|
Specific Techniques & Preparation Procedures
Specific Techniques and Preparation Procedures with Liposome AutoMaker
Principles
Multilamellar vesicles (MLVs) and small unilamellar and multilamellar vesicles (SUVs and SMVs)
are prepared by the general methods of vortex-mixing anr ultrasonication, respectively, so called
Bangham methods. Large unilamellar and multilamellar (LUVs and LMVs) are prepared by a
modified technique of reverse-phase evaporation, in which W/O emulsions are prepared by
vortex-mixing instead of bath-type ultrasonication, and bilayer vesicles can be obtained transitionally
by continuous vortex-mixing under reduced pressure. Giant unilammellar and multilamellar vesicles
(GUVs and GMVs) are prepared by osmotic pressure-dependent hydration of sugar-doped lipid film
(1,2), the former being composed of high ratio of negatively charged phospholipid (Fig.1).
Chloroform or ether can be used to dissolve lipids and oil-soluble compounds. Lipid concentrations
of less than 1 mM in 10 mM NaCl or water and more than 10mM in 100 mM NaCl could result in
unilamellar and multilamellar vesicles, respectively.
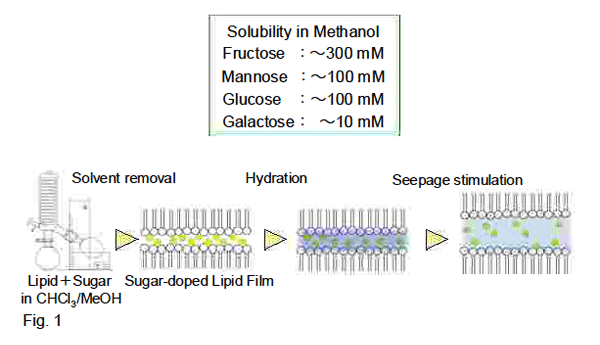
 (1) Lipid film is prepared by vortex-mixing of chloroform or ether solution of lipid at an appropriate
Hydration Seepage stimulation (1) Lipid film is prepared by vortex-mixing of chloroform or ether solution of lipid at an appropriate
Hydration Seepage stimulation
(2) A buffer solution of 10 mM Tris-HCl containing10 or 100 mM NaCl (pH 7.5) is added to the lipid
film and subjected to vortex-mixing again at the indicated temperature.
MLV Preparation
(1) Lipid ether solution D
(2) Buffer solution C
(1) Lipid ether solution
transfer
(2) Lipid film formation
(3) Buffer solution transfer
(4) Vortex-mixing
(5) Collection A
(1) SUVs are prepared from MLV by ultrasonication with a Branson sonifier Model 450 at a lipid
concentration of less than 1mM in 10 mM Tris-HCl containing 10 mMNaCl (pH 7.5) for 20 min at an
appropriate temperature.

(2) SMVs are prepared from MLV by ultrasonication with a Branson sonifier Model 450 at a lipid
concentration of more than 10 mM in 10 mM Tris-HCl containing 100 mM NaCl (pH 7.5) for 20 min
at an appropriate temperature.
SUV or SMV Preparation
(1) Lipid ether solution D
(2) Buffer solution C
(1) Lipid ether solution transfer
(2) Lipid film formation
(3) Buffer solution transfer
(4) Vortex-mixing
(5) Transfer into sonifier
(6) Ultrasonication
(7) Collection A
(1) Lipid film is prepared by vortex-mixing of chloroform or ether solution of lipid at an appropriate
temperature under reduced pressure. Lipid is added so that its final concentration is less than 1 mM
and more than 10mMin the procedures (3) and (4), respectively.
(2) Chloroform or ether is added to the lipid film and subjected to vortex-mixing again at the indicated
temperature.
(3) For LUV preparation, a buffer solution of 10 mM Tris-HCl containing 10 mM NaCl (pH 7.5) is
added to the chloroform and ether solution at a organic solvent to aqueous solution ratio of 1.2 to 1 and
less than 1, respectively, so that its final concentration is less than 1 mMand subjected to vortex-mixing
again at the indicated temperature under initially normal pressure and then. reduced pressure.
 (4) For LMV preparation, a buffer solution of 10 mM Tris-HCl containing 100 mM NaCl (pH 7.5) is
added to the chloroform and ether solution at a organic solvent to aqueous solution ratio of 1.2 to 1 and
less that 1, respectively, so that its final concentration is more than 10 mM and subjected to
vortex-mixing again at the indicated temperature under initially normal pressure and then. reduced
pressure. (4) For LMV preparation, a buffer solution of 10 mM Tris-HCl containing 100 mM NaCl (pH 7.5) is
added to the chloroform and ether solution at a organic solvent to aqueous solution ratio of 1.2 to 1 and
less that 1, respectively, so that its final concentration is more than 10 mM and subjected to
vortex-mixing again at the indicated temperature under initially normal pressure and then. reduced
pressure.
LUV or LMV Preparation
(1) Lipid ether solution D
(2) Chloroform or ether F
(3) Buffer solution C
(1) Lipid ether solution transfer
(2) Lipid film formation
(3) Ether solution transfer
(4) Buffer solution transfer
(5) Vortex-mixing under reduced pressure
(6) Collection A
GUV Preparation
(1) Lipid film is prepared by vortex-mixing of chloroform or ether solution of lipid at a high molar ratio
of negatively charged to neutral lipid at an appropriate temperature under reduced pressure. Lipid is
added so that its final concentration is to less than 1mMin the procedures (4).
(2) Chloroform or ether is added to the lipid film and subjected to vortex-mixing again at the indicated
temperature.
(3) Methanol solution of fructose is added at a fructose-to-lipid ratio of 10, and methanol is added at a
finalmethanol-to-chloroform or –ether ratio of 1 to 2 or less than 1 to 2, respectively. Then, lipid film
is again prepared by vortex-mixing of the solution at the indicated temperature under reduced pressure.
(4) Abuffer solution of 10mM Tris-HCl containing 10 mM NaCl (pH 7.5) is added to the lipid film so
that its final concentration is to less than 1 mM and subjected to gentle vortex-mixing again at the
indicated temperature.
GMV Preparation
(1) Lipid film is prepared by vortex-mixing of chloroform or ether solution of lipid at an appropriate
temperature under reduced pressure. Lipid is added so that its final concentration is more than 10mM
in the procedures (4).
(2) Chloroform or ether is added to the lipid film and subjected to vortex-mixing again at the indicated
temperature.
(3) Methanol solution of fructose is added at a fructose-to-lipid ratio of 1-10, and methanol is added at a
finalmethanol-to-chloroform or –ether ratio of 1 to 2 or less than 1 to 2, respectively. Then, lipid film
is again prepared by vortex-mixing of the solution at the indicated temperature under reduced pressure.
 (4) Abuffer solution of 10mM Tris-HCl containing 10 mM NaCl (pH 7.5) is added to the lipid film so (4) Abuffer solution of 10mM Tris-HCl containing 10 mM NaCl (pH 7.5) is added to the lipid film so
that its final concentration is more than 10 mM and subjected to gentle vortex-mixing again at the
indicated temperature.
GUV or GMV Preparation
(1) Lipid ether solution D
(2) Fructose/methanol solution F
(3) Buffer solution C
(1) Lipid ether solution transfer
(2) Fructose methanol solution transfer
(3) Lipid film formation
(4) Buffer solution transfer
(5) Moderate vortex-mixing
(6) Collection A
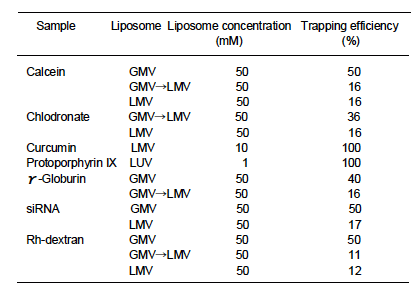
There are two incorporation techniques of a substance into liposomes: into liposome-inside
(encapsulation) and liposome membrane bilayer. Water-soluble substances are encapsulated, and
water-insoluble and oil-soluble substances are incorporated into bilayer. In general, small and large
sizes of oil-soluble substances are easy to be incorporated during and after liposome preparations,
respectively. Water-soluble substances are mixed in water phase, and oil-soluble substances in
organic solvent phase.
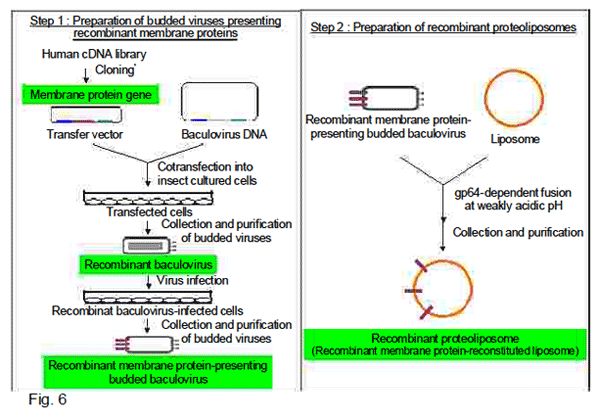
Proteoliposomes are liposomes that contain water-insoluble membrane proteins in their bilayer
membrane. Recombinant proteoliposomes are prepared as follows: first, targeted membrane proteins
are expressed on insect cell membranes using baculovirus expression systems to produce recombinant
membrane protein-presenting budded viruses and next, reconstituted in liposomemembranes by fusion
of the resulting budded viruses with liposomes (Fig. 6) (3-8).
(1) Lipid film is prepared by vortex-mixing of ether solution of lipid at an appropriate
temperature under reduced pressure. Lipid is added so that its final concentration is less than 1 mM
and 50mMfor preparation of LUV and LMV, respectively.
(2) Chloroform or ether is added to the lipid film and subjected to vortex-mixing again at the indicated
temperature.
(3) For solute-encapsulated LUV and LMV preparation, a buffer or water solution containing small
molecules, (drugs), peptide, or nucleic acid such as small DNA, siRNA and miRNA, etc.is added to the
chloroform and ether solution at a organic solvent to an aqueous solution ratio of 1.2 to 1 and less that 1,
respectively, and subjected to vortex-mixing again at the indicated temperature under initially normal
pressure and then. reduced pressure.
 (4) Solute-encapsulated LUVs or LMVs are dialyzed against an isotonic solution of 10 mM Tris-HCl
(pH 7.5) containing an appropriate amount of NaCl or other compound at 4 or the indicated
temperature for 3-4days. (4) Solute-encapsulated LUVs or LMVs are dialyzed against an isotonic solution of 10 mM Tris-HCl
(pH 7.5) containing an appropriate amount of NaCl or other compound at 4 or the indicated
temperature for 3-4days.
Encapsulation into liposomes LUV or LMV
(1) Lipid ether solution D
(2) Chloroform or ether F
(3) Substance solution C
(1) Lipid or ether solution transfer
(2) Lipid film formation
(3) Ether solution transfer
(4) Substance solution transfer
(5) Vortex-mixing under reduced pressure
(6) Collection A
(1) Lipid film is prepared by vortex-mixing of chloroform or ether solution of lipid at a high molar ratio
of negatively charged to neutral lipid for GUV or of any type for GMV at an appropriate temperature
under reduced pressure. Lipid is added so that its concentration is less than 1 mM and 50 mM for
preparation of GUV and GMV, respectively.
(2) Chloroform or ether is added to the lipid film and subjected to vortex-mixing again at the indicated
temperature.
(3) Methanol solution of fructose is added at a fructose-to-lipid ratio of 10 for GUV or 1-10 for GMV,
and methanol is added at a final methanol-to-chloroform or -ether ratio of 1 to 2 or less than 1 to 2.
Then, lipid film is again prepared by vortex-mixing of the solution at the indicated temperature under
reduced pressure.
(4) For solute-encapsulated GUV and GMV preparation, a buffer or water solution containing small
molecules, (drugs), peptide, protein such as antigen, antibody or enzyme or nucleic acid such as small
DNA, siRNA and miRNA, etc. is added to the film, and subjected to gentle vortex-mixing again at the
indicated temperature.

(5) Solute-encapsulated GUVs or GMVs are dialyzed against an isotonic solution of 10 mM Tris-HCl
(pH 7.5) containing an appropriate amount of NaCl or other compound at 4 or the indicated
temperature for 3-4days.
Encapsulation into liposomes GUV or GMV
(1) Lipid ether solution D
(2) Fructose/methanol solution F
(3) Substance solution C
(1) Lipid ether solution transfer
(2) Fructose methanol solution transfer
(3) Lipid film formation
(4) Substance solution transfer
(5) Moderate vortex-mixing
(6) Collection A
Incorporation into lipid bilayer
(1) Lipid film is prepared by vortex-mixing of chloroform or ether solution of lipid and water-insoluble
small molecule (drug), peptide, PEG-PE, magnetic compound, etc. at an appropriate temperature under
reduced pressure.
(2) Liposomes such as MLV, SU(M)V, LU(M)V, and GU(M)V are prepared as described in 1A.
 LUV or LMV LUV or LMV
(1) Lipid ether solution D
(2) Substance/organic solvent solution E
(3) Chloroform or ether F
(4) Buffer solution C
(1) Lipid ether solution transfer
(2) Substance/organic solvent solution transfer
(3) Lipid film formation
(4) Chloroform or ether solution transfer
(5) Buffer solution transfer
(6) Vortex-mixing under reduced pressure
(7) Collection A
GUV or GMV
(1) Lipid ether solution D
(2) Substance/organic solvent solution E
(3) Fructose/methanol solution F
(4) Buffer solution C
(1) Lipid ether solution transfer
(2) Substance/organic solvent solution transfer
(3) Fructose methanol solution transfer
(4) Lipid film formation
(5) Buffer solution transfer
(6) Moderate vortex-mixing
(7) Collection A
(1) Liposomes such as MLV, SU(M)V, LU(M)V, and GU(M)V are prepared as described in 1A.
(2) Each of water-insoluble small molecule (drug), peptide, etc. dissolved in organic solvent such as
ethanol, DMSO, etc. is added to the liposomes at a ratio of 3%(v/v) to the solution, and reaction
mixture is kept at RT for about 1h.
(3) For preparation of recombinant proteoliposomes, budded viruses presenting recombinant
membrane proteins are added to the liposomes composed of PS/PC(1:1), the pH of the mixture is
adjusted to pH 4 by adding acetate buffer, the mixture is kept at RT for 1h, and Tris buffer is added to
return its pH to neutral.
Image below shows fluorescence microscopic image of giant liposomes, inot which protoporphyrin IX molecules dissolved in DMSO are incorporated after liposome preparation.
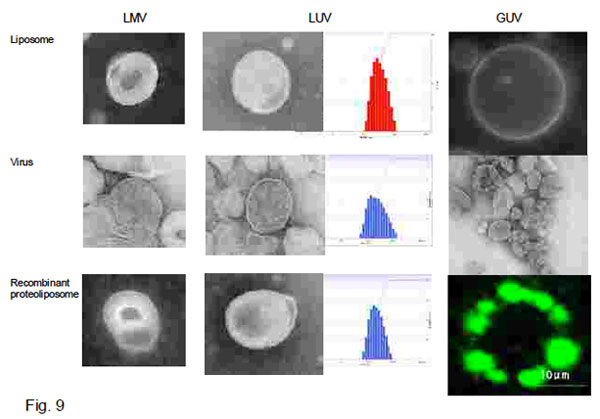
Fig. 9 shows electron microscopic images and size distribution of LMV, LUV, virus and their
recombinant proteoliposomes and fluorescent microscopic images of GUV and its recombinant
proteoliposome.
 LUV or LMV LUV or LMV
(1) Lipid ether solution D
(2) Substance/organic solvent solution E
(3) Ether F
(4) Buffer solution C
(1) Lipid ether solution transfer
(2) Lipid film formation
(3) Ether solution transfer
(4) Buffer solution transfer
(5) Vortex-mixing under reduced pressure
(6) Substance/organic solvent solution transfer during vortex-mixing of LUV (LMV)
(7) Collection A
GUV or GMV
(1) Lipid ether solution D
(2) Substance/organic solvent solution E
(3) Fructose/methanol solution F
(4) Buffer solution C
(1) Lipid ether solution transfer
(2) Fructose methanol solution transfer
(3) Lipid film formation
(4) Buffer solution transfer
(5) Moderate vortex-mixing
(6) Substance/organic solvent solution transfer during vortex-mixing of GUV (GMV)
(7) Collection A
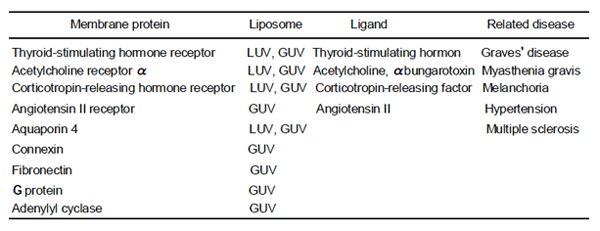
Water-soluble substances are bound to pre-formed liposomes noncovalently or covalently. For
noncovalent binding, electrostatic or hydrophobic interaction between ligand and liposome membrane
surface is critical, and for covalent binding, bifunctional reagent are required between them.
(1) Liposomes such as MLV, SU(M)V, LU(M)V, and GU(M)V are prepared as described in 1A.
(2) Each of water-soluble small molecule (drug), peptide, protein, nucleic acid, etc. is added to the
liposomes at an appropriate ratio to the solution, and reaction mixture is kept at RT for about 1h.
Electron microscopic images of LUV, on the surface of which proteins are bound.
 LUV or LMV LUV or LMV
(1) Substance/buffer solution B
(2) Lipid ether solution D
(3) Chloroform or ether F
(4) Buffer solution C
(1) Lipidether solution transfer
(2) Lipid film formation
(4) Chloroform or ether solution transfer
(5) Buffer solution transfer
(6) Vortex-mixing under reduced pressure
(7) Substance buffer solution transfer with vortex-mixing of LUV(LMV)
(8) at RT for 1h
(9) Collection A
GUV or GMV
(1) Substance/buffer solution B
(2) Lipid ether solution D
(3) Fructose/methanol solution F
(4) Buffer solution C
(1) Lipid ether solution transfer
(2) Fructose/methanol solution transfer
(3) Lipid film formation
(4) Buffer solution transfer
(5) Moderate vortex-mixing
(6) Substance/buffer solution transfer with vortex-mixing of GUV(GMV)
(7) at RT for 1 h
(8) Collection A
(1) Liposomes such as MLV, SU(M)V, LU(M)V, and GU(M)V are prepared as described in 1A.
(2) SFB-modified liposomes are prepared by adding a DMSO solution of SFB (succunumidyl 4-formylbenzoate) to liposomes composed of PS/PC/PE(5:1:4) in 10mM HEPES containing 100mM
NaCl
(pH 7.5) and after 3 h at 27 , reaction mixture is dialyzed overnight against 10mM MES
containing 100mM NaCl (pH 4.7)
(3) SANH-modified protein or peptide is prepared by adding a DMSO solution of SANH
(succunumidyl 4-hydrazinonicotinate acetone hydrazone) to protein or peptide in 10mM HEPES
containing 100mM NaCl
(pH 7.5) and after 1 h at 27 , reaction mixture is dialyzed overnight against
10mM MES containing 100mM NaCl (pH 4.7)
(4) Protein- or peptide-bound liposomes are prepared by reacting SANH-modified protein or peptide
with SFB-modified liposomes at 27 for 3h and returning its pH to neutralwith 1MTris.
The outlone of the preparation procedure is shown in Fig. 10.
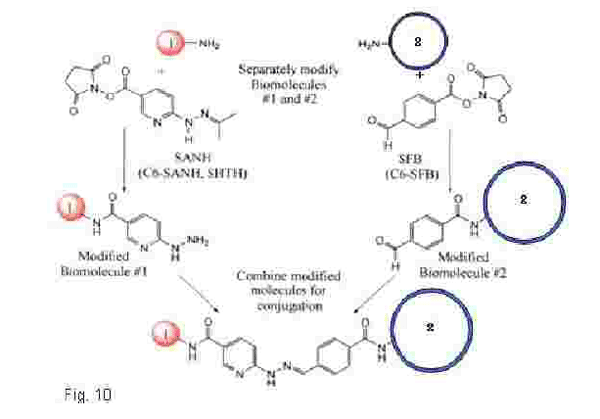
Table shows the results on the binding of proteins with GMV using the bifuntional reagents, SANH and SFB
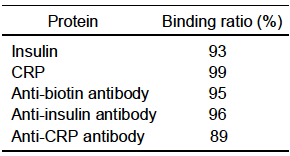
 SFB-GUV or -GMV SFB-GUV or -GMV
(1) Lipid ether solution D
(2) SFB/DMSO solution E
(3) Fructose/methanol solution F
(4) Buffer solution C
(1) Lipid ether solution transfer
(2) Fructose methanol solution transfer
(3) Lipid film formation
(4) Buffer solution transfer
(5) Moderate vortex-mixing
(6) SFB/DMSO solution transfer during vortex-mixing of GUV(GMV)
(7) RT for 1 h
(8) Collection A
(9) Dialysis against MES buffer (pH4.7)
SANH-Peptide or -Protein
(1) Peptide or protein solution B
(2) SANH/DMSO solution E
(3) Buffer solution C
(1) Peptide or protein solution transfer
(2) SANH/DMSO solution transfer during vortex-mixing of peptide(protein) solution
(3) RT for 1 h
(4) Collection A
(5) Dialysis against MES buffer (pH4.7)
Peptide (Protein) GUV (GMV)
(1) SANH-peptide or -protein solution B
(2) SFB-GUV or –GMV solution C
(3) 1M Tris D
(1) SFB-GUV or -GMV solution transfer
(2) SANH-peptide or -protein solution transfer during vortex-mixing of SFB-GV solution
(3) RT for 1 h
(4) 1M Tris
(5) Collection A
Principles:
Sometimes, vortex-mixing or vortex-mixed liposomes are required to intermix with ultrasonicating
membrane vesicles, and vice versa. This is only achieved with the automated equipment and useful
for preparation of ultrasonicated proteoliposomes available for liposome vaccines (9,10).
Techniques:
(1) MLVs are prepared as described in 1A.
(2) Formalin-inactivated bacteria or viruses are ultrasonicated with a Branson, and during ultrasonication, MLVs are added to the suspension and further ultrasonicated at low temperature for 20
min, resulting in proteoliposomes available for liposome vaccine
Applications:
Fig. 12 shows electron microscopic images and size distribution of SUV, ultrasonicated bacteria and
the resultong proteoliposomes.
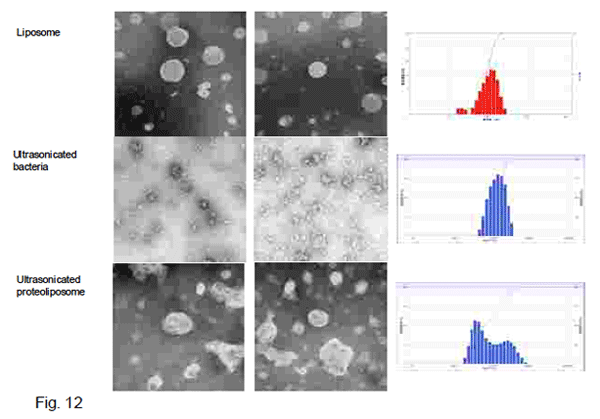
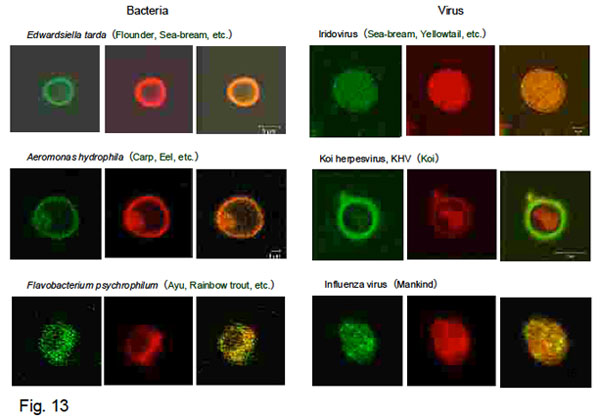
Fig. 13 shows confocal laser fluorescence microscopic images of successful liposome vaccines
prepared by ultrasonication of bacteria or viruses with MLV.
Lipsosome Vaccine Preparation
(1) Lipid/chloroform or ether solution D
(2) Inactivated-bacteria or -virus suspension B
(3) Buffer solution C
(1) Inactivated-bacteria or -virus suspension transfer to sonifier
(2) Ultrasonication
(3) Lipid/chloroform or ether solution transfer
(4) Lipid film formation
(5) Buffer solution transfer
(6) Vortex-mixing
(7) Transfer to sonifier during ultrasonication of bacteria or virus
(8) Ultrasonication
(9) Collection A
|


 (1) Lipid film is prepared by vortex-mixing of chloroform or ether solution of lipid at an appropriate
Hydration Seepage stimulation
(1) Lipid film is prepared by vortex-mixing of chloroform or ether solution of lipid at an appropriate
Hydration Seepage stimulation